
- #ATOMS TO MASS IN GRAMS CONVERTER CALCULATOR HOW TO#
- #ATOMS TO MASS IN GRAMS CONVERTER CALCULATOR SERIES#
The kilogram (SI unit symbol: kg) is the base unit of mass in the Metric system and is defined as being equal to the mass of the International Prototype of the Kilogram.
#ATOMS TO MASS IN GRAMS CONVERTER CALCULATOR HOW TO#
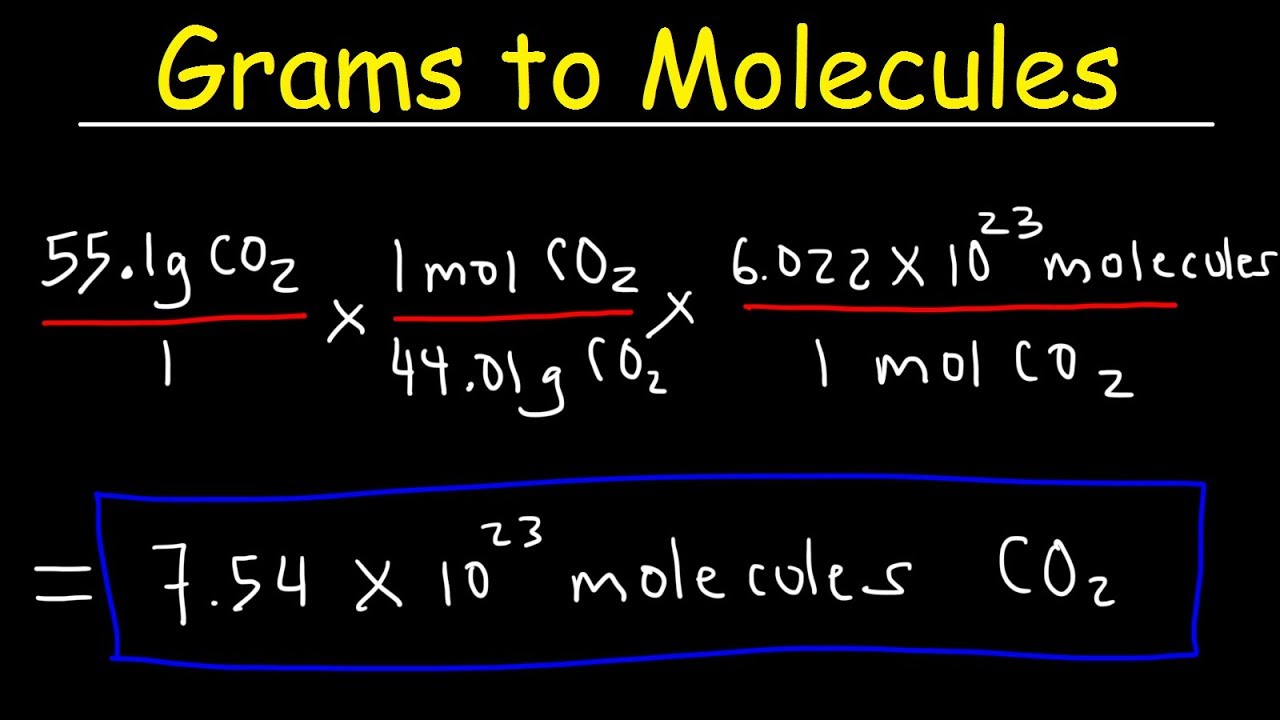
How to convert Atomic Mass Unit to Kilograms (u to kg)?ġ x 1.660540199E-27 kg = 1.660540199E-27 Kilograms.Īlways check the results rounding errors may occur. The base unit for mass weight is kilograms (SI Unit) Office of Public Sector Information.Conversion: Atomic Mass Unit to Kilograms The International System of Units (SI) (8th ed.). International Bureau of Weights and Measures (2006).“The ontological distinction between units and entities”. “The Atomic Mass Unit, the Avogadro Constant, and the Mole: A Way to Understanding”. “Determination of the Avogadro Constant by Counting the Atoms in a 28Si Crystal”. Molar mass of KMnO 4 = 39.01 + 54.94 + (16.00)(4) = 157.95 g/molĭivide the mass in grams by the molar mass (g/mol) and get moles: Using a periodic table, look up the masses of the elements: Divide the mass by the molar mass for an answer in moles.įor example, find the number of moles in 25.0 grams of potassium permanganate (KMnO 4).Start with the number of grams and the chemical formula.Once again, you use the molar mass of the substance. Grams of H 2O 2 = 0.700 moles x 34.014 grams/mole = 23.810 grams Grams to Moles ConversionĬonverting grams to moles is just as easy. This chemistry video tutorial explains the conversion process of grams to atoms which is useful in solving common stoichiometry practice problems.
#ATOMS TO MASS IN GRAMS CONVERTER CALCULATOR SERIES#
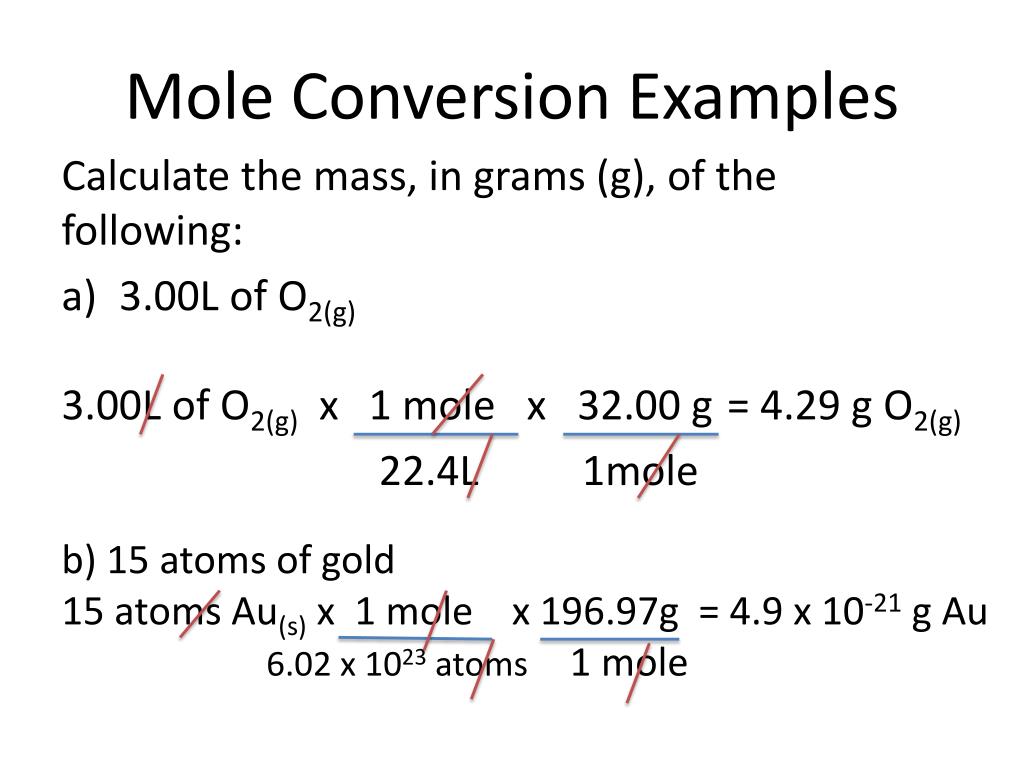
Or you can choose by one of the next two option-lists, which contains a series of common organic compounds (including their chemical formula) and all the elements. Now, multiply the moles of hydrogen peroxide by its molar mass for the answer in grams: This online calculator you can use for computing the average molecular weight (MW) of molecules by entering the chemical formulas (for example C3H4OH (COOH)3 ). Write down the atomic masses of the elements: You have 3 significant figures in 0.700 moles. This time let’s pay attention to the number of significant figures. Mass of 3.6 moles H 2SO 4 = 3.6 moles x 98.076 grams/mole = 353.07 grams Moles to Grams Example #2įind the mass in grams of 0.700 moles of hydrogen peroxide (H2O2).
Add up the masses of each element according to the chemical formula. Molar Mass Calculator Chemical Formula C also known as Carbon Molar Mass of C: 12.01 g/mol If you have a sample that contains only atoms of a particular element, weigh the sample in grams and divide by the atomic weight of the element. Remember, if there is no subscript, it’s the same as multiplying by 1. The atomic weight of carbon is 12 atomic mass units (amu), so the weight of one mole is 12 grams. You multiply the atomic mass of each element by its subscript. Read more about gram weight conversions at the bottom The procedure to use the grams to atoms calculator is as follows: Step 1: Enter the atomic mass number. Formula weight (F.W.) is the sum of the atomic weights of all atoms in a given. Now, use the chemical formula and find the molar mass of H 2SO 4. The mass molarity calculator tool calculates the mass of compound. This gives an answer in grams.įor example, find the mass in grams of 3.6 moles of sulfuric acid (H 2SO 4).įirst, look up the atomic masses of the elements:
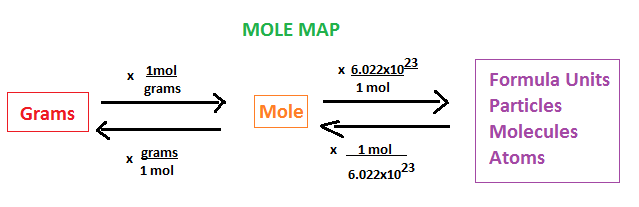
How to Convert Moles to GramsĪll you need is a periodic table for looking up the atomic masses of the elements. The conversion is easy, providing you set it up right. Chemical equations relate quantities of reactants and products using moles, yet you measure chemicals on scales and balances, which use grams. This entry was posted on Augby Anne Helmenstine (updated on August 19, 2022)ĭo the moles to gram conversion by multiplying the number of moles by the molar mass of the element or compound.Ĭonverting moles to grams or grams to moles is something you do all the time in chemistry.


 0 kommentar(er)
0 kommentar(er)
